In short
- Algae blooms are a global problem that worsens each year;
- Ultrasonic treatment has the power to effectively control these harmful blooms;
- Not all ultrasonic algae control technologies are the same.

In recent years, various innovations regarding lake restoration and algal bloom remediation have been introduced and tested. One of these methods is using interactive ultrasonic programs that control algae growth.
With every new technology, it is important to study the effect on algae and that of the ecosystem it is introduced into. This blog addresses the most common concerns regarding the use of ultrasound in lakes/reservoirs based on research conducted by various academic institutions.
Developed by European universities

The Chameleon Technology™ for algae control, also known as Monitor-Predict-Control (MPC) technology, was developed based on a large research grant from the European Commission[1]. The technology resulted from the collaboration between:
- The KAUNO Technological University;
- The Hellenic Centre for Marine Research;
- The Technological Institute of Norway;
- The Meteorological Institute of Poland, and
- a group of industry partners.
The results of these studies is the MPC-Buoy technology, a solar-powered buoy that uses in-situ, real-time water quality monitoring to identify and predict algal growth and autonomously applies specific ultrasonic frequencies to match the algal type present in the water.

At the basis of the research was establishing a database that contains algal types, water quality data, and matching ultrasonic parameters.
This database operates an algorithm that drives ultrasound program changes in the MPC-Buoy. This database is automatically updated with every new reading collected from the MPC-Buoys operating worldwide.
Manipulating light to reduce cyanobacteria
When a water body suffers from harmful algal blooms, solutions are most often sought in the form of reducing phosphate – the key nutrient that algae and cyanobacteria consume to grow. However, aside from phosphate, light is also a critical resource for all phytoplankton.
Manipulating light as a way to reduce cyanobacteria is a feasible method that has been recently explored by the University of Missouri (MO) and University of Kansas (KS) in the United States. They found that reducing the photosynthetic active radiation (PAR) in a water body with 60.4 – 86.4% resulted in a 77.9% decline in cyanophyte biovolume in tanks dominated by cyanobacteria, which were predominantly affected by the limited light availability.
Additionally, these studies show the control of algal growth while keeping phosphate levels stable throughout the experiment.[2]
LG Sonic’s MPC-Buoy works through a similar principle. Instead of limiting nutrients, it reduces the light availability to disrupt the growth of cyanobacteria and other blooming algae. Other studies, such as the one performed by the University of Aarhus in Denmark, showed that PAR declines exponentially over depth in both shallow and deeper water bodies[3].
Therefore, instead of reducing light penetration into the top layer of the water surface, it’s also possible to bring algal cells towards the water depth, where levels of PAR are sufficiently reduced.
Affecting algae’s buoyancy
The MPC-Buoy uses ultrasound as a mechanism to accomplish this. Ultrasound can be represented as an added hydrostatic pressure onto the algal cell, affecting the buoyancy of the cell. The algae require cell buoyancy to remain in the upper layer of the water and obtain sufficient light to bloom.
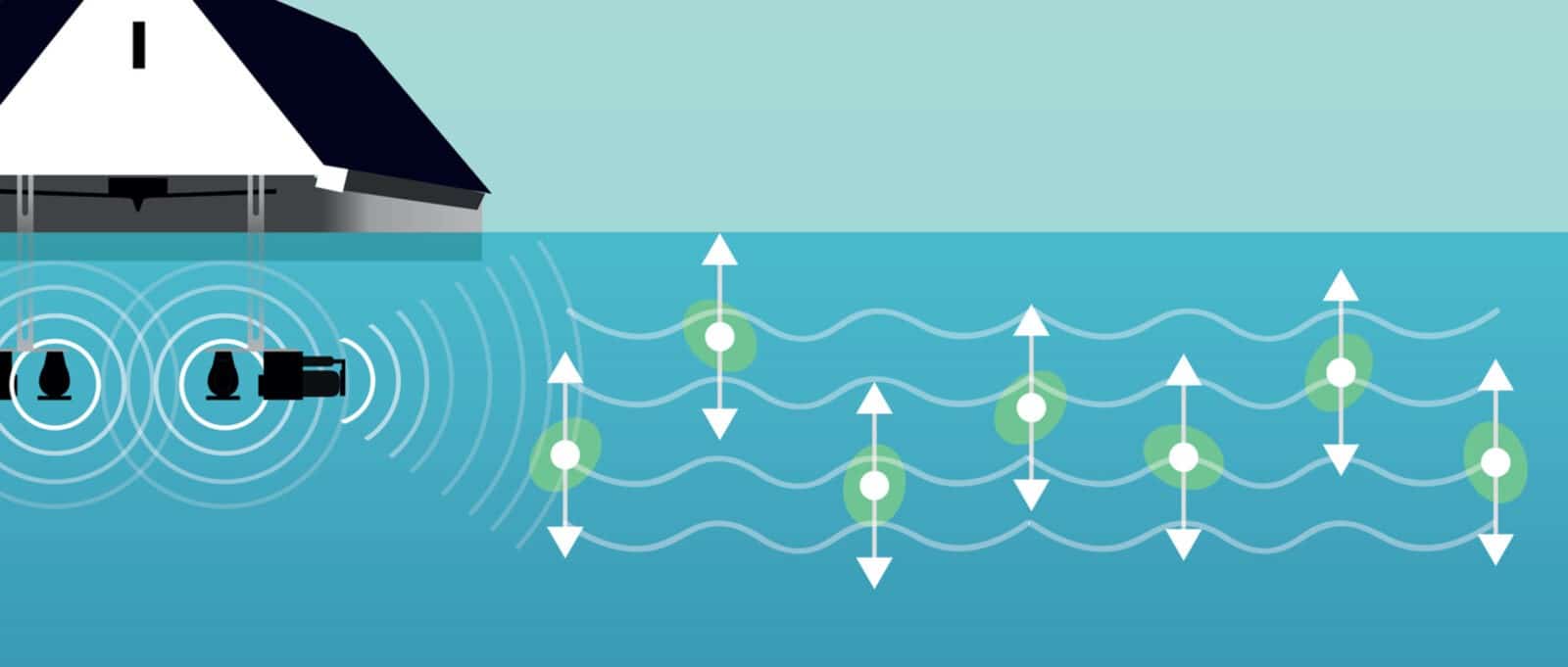
It is commonly thought that algal buoyancy is a matter of generation and collapse of gas vesicles, which is only partially true. Collapse of gas vesicles is irreversible and for some algal types, not possible due to the size and shape of the vesicle.
Instead, the vertical migration of these algae is a more complex function. The lift provided by gas vesicles counteracts cell ballasts, such as carbohydrate and protein, which can be more accurately regulated based on the present water quality (turbidity, phosphate and nitrate concentrations) [4], as well as hydrodynamic variations such as hydrostatic pressure, waterflow and mixing of the surface column.
This is the area in which the ultrasound plays a key role, as the ultrasonic waves form a continuous pressure cycle around the algal cells, affecting the buoyancy regulation, which does not allow them to obtain sufficient buoyancy to remain the area where PAR is high enough for them to bloom.
This also explains the requirement for frequent variations in ultrasonic parameters such as amplitude, frequency, and waveform, as buoyancy regulation is a continuous process of algae closely related to their photosynthetic activities. It is, therefore, important that the hydrostatic pressures around the algae continuously change.
American Water’s research
This theory was tested in 2014, by Dr. Orren D. Schneider and Lauren A. Weinrich. They performed in-situ tests with 4 MPC-Buoy systems in a drinking water reservoir in New Jersey for a period of 5 months.

Water samples where taken throughout the entire test period and algal species were determined. They found during the initial part of the study, algae were controlled well by the MPC-Buoys. However, on August 13, an inlet from an untreated reservoir was opened because additional water was needed at the plant. This algae-laden water entered Reservoir 1 and seeded the reservoir with Aphanizomenon, a filamentous cyanobacterium.
Almost immediately, increased levels of algae, organic carbon and taste- and odor molecules where detected at the plant intake. Aphanizomenon started blooming in the reservoir until the ultrasonic program was changed matching this algal species. [5]
Why control algae if nutrients are the problem?
Algal blooms are often caused by eutrophication, a process in which nitrogen and phosphorous loads in a water body increase to a point that algae and plants start growing excessively. Once algae start blooming, it leads to increased turbidity, chlorophyll a, pH, and phycocyanin. These parameters have a negative effect on the growth-rate of submerged macrophytes and other aquatic organisms.
Submerged macrophytes play a key role in the clarity of the water within an ecosystem; first because they consume nutrients that would otherwise be available for algal growth, and also because submerged aquatic plants provide shelter for zooplankton against fish.
Zooplankton are natural phytoplankton grazers; hence, when zooplankton disappears, the water loses its clarity due to excessive phytoplankton growth. Another important effect of reduced plant growth and water quality is on levels of Dissolved Oxygen (DO) within a water body, which can become unstable, and the deepest water levels (hypolimnion) can become anoxic (lack of oxygen). Thus, the microbial aerobic digestion in the deeper water layers of nutrients become compromised.
When algae blooms
The blooming of algae in a water body affect the entire production chain in water. In the hypolimnion, anoxic conditions lead to a reduced organic decomposition. This means that the sediment layer continues to grow. Due to lack of oxygen, it may release its content (nutrients, metals) into the water column, often leading to a further increase in algal proliferation.

Under these conditions, reducing nutrient inflow will not alter any of the water quality parameters mentioned before. On the contrary, the sediment will continue to release nutrients as long as algae are blooming, and the water quality will further deteriorate.
Therefore, to actively solve the problem of algal blooms, it is important that algal proliferation is first controlled so the water quality can stabilize, allowing the aquatic ecosystem to adjust.
Ultrasonic effects on non-target organisms
Cavitation is another important concept of ultrasonic waves. Cavitation is where ultrasound or other hydrostatic pressure in a liquid causes the formation of micro-bubbles that implode, causing intense heat, pressure, micro-jets and the formation of hydrogen free radicals.
Cavitation is used in a wide variety of industrial processes, such as cleaning of jewelry, sterilization of surgical equipment, homogenization of (cell) samples and lysing of cells for laboratory applications.
Cavitation is often characterized by using high frequencies into the range of MHz. Although the forms and characteristics of the cavitation may differ, it can be generated both at low (kHz) and high (MHz) frequencies, but primarily depends on output power.
Lowe & L. Brand [6] reflect in their paper on the energy requirement to generate cavitation in water, and Joyce et al. (2010)[7] cited cavitation as a primary cause of algae control within their experiment (power per volume range of 0.0015–0.17W/cm3).
It is, therefore, assumed that any study with a power per volume range greater than this is utilizing cavitation. However, it is good to know that other factors within an experiment also contribute to the generation of cavitation. Ultrasound utilized by the products manufactured by LG Sonic, generally uses power volumes of 0.55*E-8 and 0.33*E-6 respectively, which is well under the threshold of generating cavitation.
However, many scientific studies involving ultrasound have based their methods on technologies generating cavitation, which typically use low volumes of water and equipment with high amplitudes well within the range of generating cavitation.
As far as LG Sonic is concerned, these applications would damage non-target organisms as well as algae, but could not be upscaled to a real environment, as it would require too much energy and consume ultrasonic transducers.
Effects of ultrasound on fish
De Lange (2007) conducted a study on ultrasonic systems used for algae control in surface waters and their effects on several fish species: bream, bass, common roach (Rutilus rutilus), silver bream, ruffle (Gymnocephalus cernua), common rudd (Scardinius erythrophthalmus), tenches (Tinca tinca), and pikes (Esox lucius).
The study was performed by connecting two identical basins stocked with fish through a tunnel where the fish where able to migrate from one basin to the other. De Lange used fish migration, as well as the size of the fish, as parameters to indicate stress or other effects of the ultrasonic systems.

The fish population was sampled before ultrasound was activated, after one month and after 4 months. Results showed that the fish population was evenly distributed across the two basins even after 4 months of ultrasound operation, revealing that ultrasound has no effect on fish migration between the two basins. The length of the fish between basins did not show any significant differences either.
De Lange also did not find any increased fish mortality in the experiment; therefore, it was concluded that the ultrasonic system was not noticeable or considered unsafe for the tested fish species.[8]
Griessler Bulc et al. (2011) studied a water treatment system for common carp (Cyprinus carpio) which included ultrasound. They used equipment produced by LG Sonic for their tests, which was performed in 2 fish ponds: one with the ultrasonic unit and the other without. They concluded that with treatment (including ultrasound), rearing conditions for common carp were better with higher body weight increase and higher fish production than in the pond without treatment. [9]

Krivograd Klemenčič & Griessler Bulc performed more tests on aquaculture with ultrasonic systems in 2013 [10] and 2015 [11], both on lab scale and in a fish pond. Both tests did not show any adverse effects on the fish, while the study inside the fishpond did show higher fish yield compared to the untreated pond.
Techer et al. studied the effects of ultrasonic irradiation on fish in 2017 using a commercially available low-power ultrasonic generator. Their study focused on long-term effects on the common carp (Cyprinus carpio), using 2-year-old carp that were exposed to low-power ultrasound for 30 days. These results showed that carp were unaffected by ultrasound exposure when exposed in floating cages in fishponds over a 30-day period. [12]
Effects of ultrasound on zooplankton
The university of Ljubljana performed field and lab tests on Daphnia magna using equipment manufactured by LG Sonic. In the lab-scale tests, a set of adult and juvenile Daphnia magna where exposed to ultrasound and mobility was tested after 0, 4 and 19 hours of exposure.
In both tests, no significant differences were found in the mobility of the species after 19 hours, indicating that there was no acute effect of ultrasound exposure on the tested organisms.
In the field test, the ultrasonic system was placed in a pond, and zooplankton was sampled at 0, 12, 24 and 48 hours using a conical plankton net. Two different sampling sites were used, located directly in front of the ultrasonic system, as well as 7 meters from the ultrasonic system. The samples where analyzed, determining the total number of zooplankton and the taxa.
A slight increase in zooplankton was found close to the ultrasonic transmitter. However, no significant differences are noticed before sonication (t0) and at different times of sonication, including after 48 h of sonication. The differences are likely due to fish predation or movement of the zooplankton. [13]

In 2017, the aquatic research and consultancy Ecofide studied the effects of the MPC-Buoy systems, produced by LG Sonic. This study was commissioned by the Zoetermeer municipality and Rijnland Water Board to research the effects of the used ultrasonic technology on zooplankton.
For this study, lab-bread zooplankton were introduced in cages for a period of 7 days. After 7 days, the number of surviving water fleas and the number of produced young were counted. This testing method is and has been applied often to field research regarding the effects on zooplankton.
For example, the Delfland Water Board has been using this method for years to determine the effects of pesticides (for example see Ecofide overview report 2008), as well as in the current Waternet research (Simoni, part of Ecological Key Factor toxicity); these field experiments form an important part (van der Oost et al., 2017; Stowa, 2016).
Cages were placed at different distances from the ultrasonic transmitters, the closest cages where placed under the buoy right in front of the transmitters, while the furthest transmitters were placed at the lake shore. Both the survival of the mature water fleas, as well as the reproduction (number of young) after 7 days, have been tested.
No negative effects caused by ultrasonic sound waves have been found regarding the survival of water fleas and the average survival rate over all samples has been 95-100%. [14]
References
[1] The research leading to these results has received funding from the European Union Seventh framework Programme ([FP7/2007-2013] [FP7/2007-2011]) under grant agreement n° [FP7-SME-2011-286875-CLEARWATERPMPC].
[2] Gaskill JA, Harris TD and North RL (2020) Phytoplankton Community Response to Changes in Light: Can Glacial Rock Flour Be Used to Control Cyanobacterial Blooms? Front. Environ. Sci. 8:540607. doi: 10.3389/fenvs.2020.540607
[3] Gonçalves-Araujo R and Markager S (2020) Light in the Dark: Retrieving Underwater Irradiance in Shallow Eutrophic Waters From AC-S Measurements. Front. Mar. Sci. 7:343. doi: 10.3389/fmars.2020.00343
[4] Zhaosheng Chu, Xiangcan Jin, Bo Yang, Qingru Zeng, Buoyancy regulation of Microcystis flos-aquae during phosphorus-limited and nitrogen-limited growth, Journal of Plankton Research, Volume 29, Issue 9, September 2007, Pages 739–745.
[5] Schneider, O.D., Weinrich, L.A. and Brezinski, S. (2015), Ultrasonic Treatment of Algae in a New Jersey Reservoir. Journal ‐ American Water Works Association, 107: E533-E542.
[6] Lowe M. 2011. Ultrasonic control of algae in stormwater systems. Water New Zealand, 7th South Pacific Stormwater Conference.
[7] Joyce, E. M., Wu, X., & Mason, T. J. (2010) ‘Effects of ultrasonic frequency and power on algae suspensions’ Journal of Environmental Science and Health, Part A, 4, 7, 863 – 866.
[8] De Lange M.C. 2007. Control of blue algae with ultrasound – the effects on fish. Report: VA2007_28. VisAdvies BV, Utrecht, 10 pp.
[9] Griessler Bulc T., Istenič D., Krivograd Klemenčič A. 2011. The efficiency of a closed-loop chemical-free water treatment system for cyprinid fish farms. Ecological engineering 37, 873-882.
[10] Krivograd Klemenčič A., Griessler Bulc T. 2013. The efficiency of constructed wetland and ultrasound for water reuse in a closed-loop small-scale cyprinid fish farm. Fresenius environmental bulletin, 22(10), 2828-2835.
[11] Krivograd Klemenčič A., Griessler Bulc T. 2015. The use of vertical constructed wetland and ultrasound in aquaponic systems. Environmental science and pollution research international, 22(2), 1420-1430
[12] Techer D., Milla S., Banas D. 2017. Sublethal effect assessment of a low-power and dual-frequency anti-cyanobacterial ultrasound device on the common carp (Cyprinus carpio): a field study. Environmental Science and Pollution Research 24(6), 5669–5678.
[13] Krivograd Klemenčič, Griessler Bulc, Pflieger. 2018. The effects of the LG Sonic technology on toxin degradation and on the environment (without algae). UNIVERSITY OF LJUBLJANA.
[14] Rineke Keijzers & Jaap Postma, Ecofide. Field research regarding possible effects of ultrasonic sound on zooplankton (2017)
